|
Porphyrins are a unique class of
aromatic tertrapyrroles that are capable
of performing diverse roles in natural
processes such as oxygen carriage,
electron transport, etc. Due to its
functional versatility, it remains the
most abundant and studied “pigment of
life”. Corroles, a type of contracted
porphyrin have recently gained a lot of
research interest for its interesting
structural and spectroscopic properties.
The name corrole was chosen by Johnson and
Price due to its strong structural
resemblance with the naturally occuring
corrin ring of vitamin B12. Because of the
similarity of the corroles with the 18
π-electron aromatic porphyrins, corroles
are better designated as intermediates
between corrins and porphyrins (Figure 1).
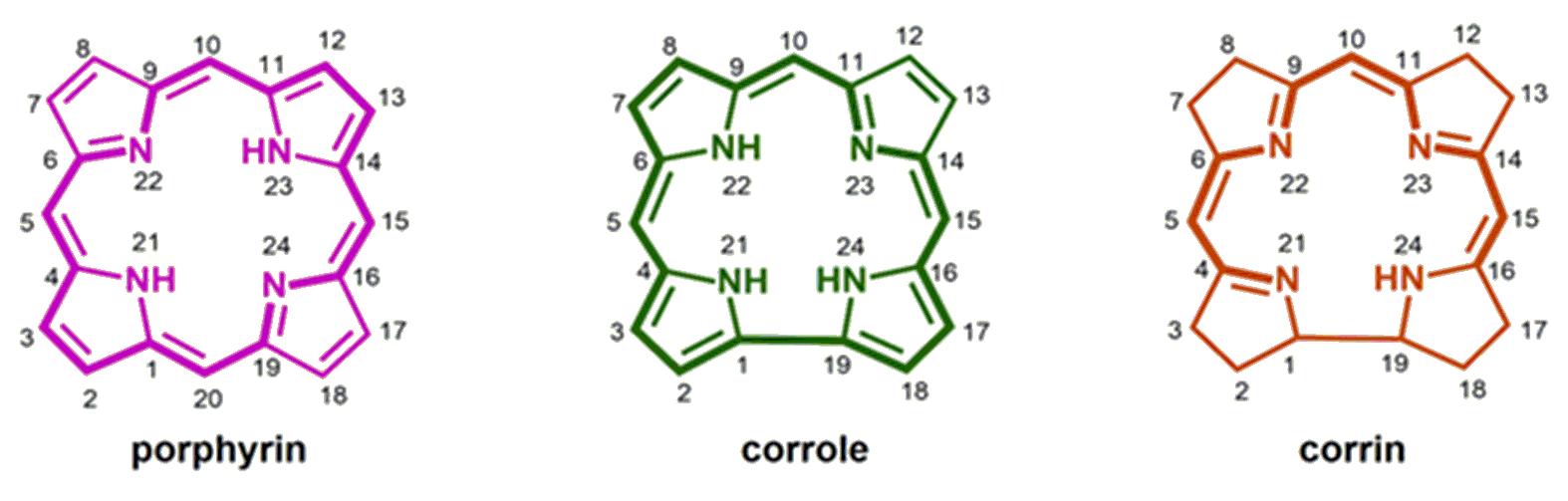
Figure 1
Comparative view of the structures of
porphyrin, corrole and corrin.
1. Our research interest
includes elucidating the mechanism of the
interaction of NO• with hemoproteins and
their model compounds.
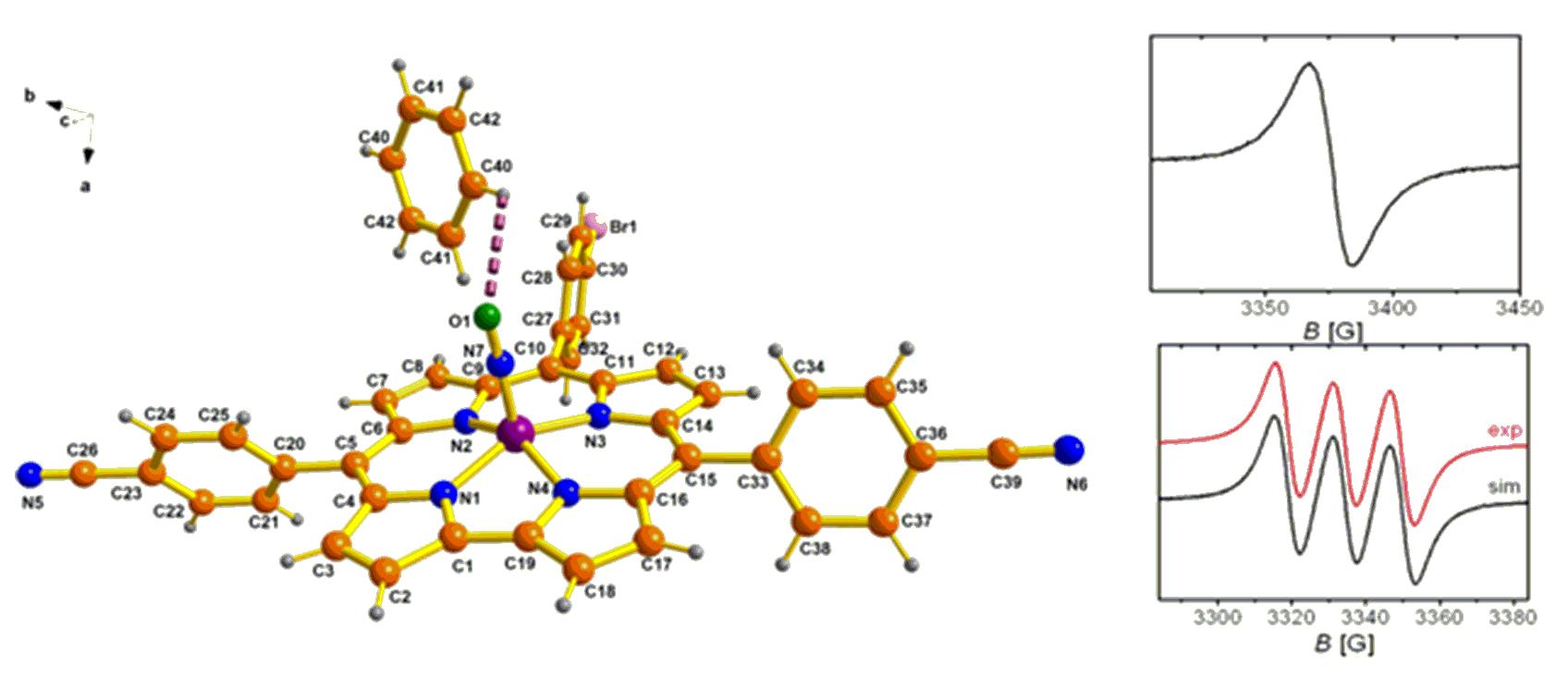
Figure 2
(a) Single-crystal X-ray structure of a
representative (cor)(FeNO)6 complex, X-band
EPR spectrum of (b) one-electron oxidised form
and (c) one-electron reduced form of a
representative complex with simulation
generated by in-situ electrolysis at 295 K in
CH2Cl2/0.1 M Bu4NPF6.
2. Our
research interest includes the generation of
corrole/metallocorrole nanostructures (e.g.,
nano sphere, nano tubes, etc.) and their
efficient uses for various device
fabrications
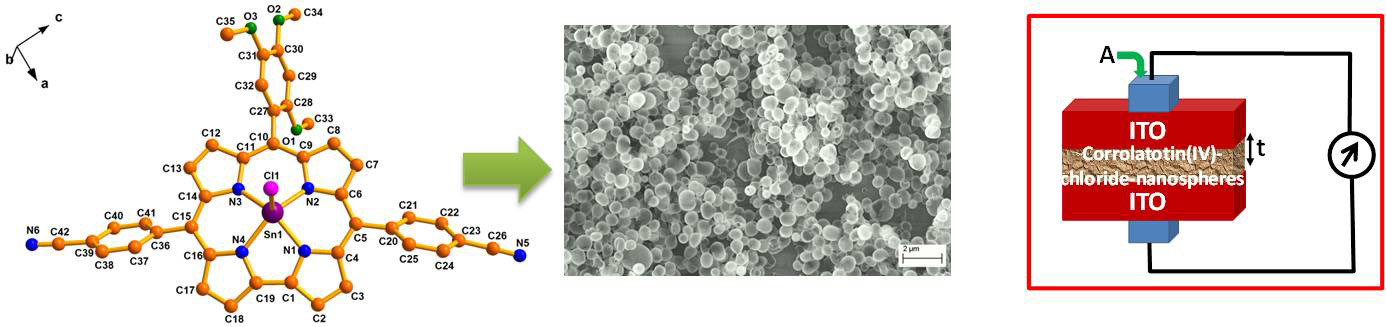
Figure 3 A flow-chart
representation depicting the application of
the nanospheres generated from a
representative corrolato tin(IV) chloride in
device fabrication; (a) single-crystal X-ray
structure of a representative corrolato
tin(IV) chloride, (b) SEM image of the
nanospheres and (c) schematic representation
of the device (Ag/ITO/nanospheres/ITO/Ag).
3. Our research interest includes
the discoveries of efficient synthetic
methodologies
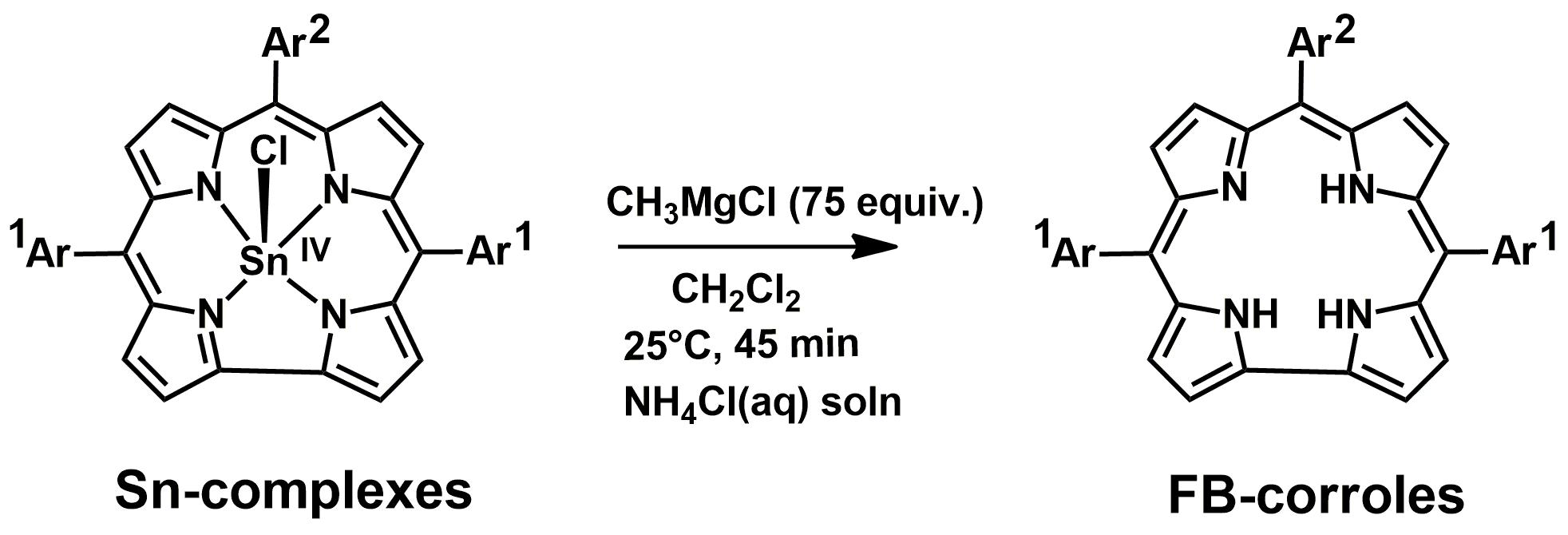
Figure 4 Demetalation
reaction of corrolato tin(IV) chlorides.
4.
Our research interest includes
stabilization of unusual oxidation states
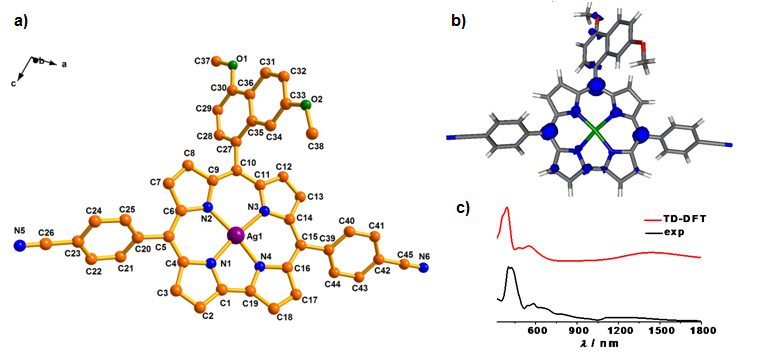
Figure 5 (a)
Single-crystal X-ray structure, (b) Spin
density representation for one-electron
oxidised form and (c) UV-Vis-NIR spectra of
one-electron oxidised form in CH2Cl2:
TDDFT-based electronic absorption spectra (──)
and experimentally obtained electronic
absorption spectra (──) of a representative
silver(III) corrolato complex.
|

