Research Topics
Experimental and Computational Methods
|
Research Overview
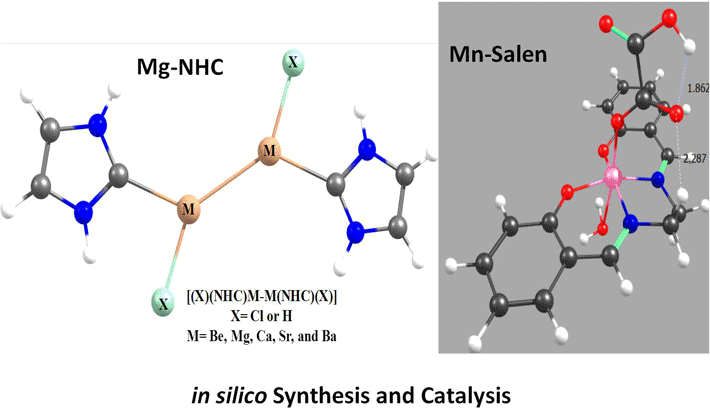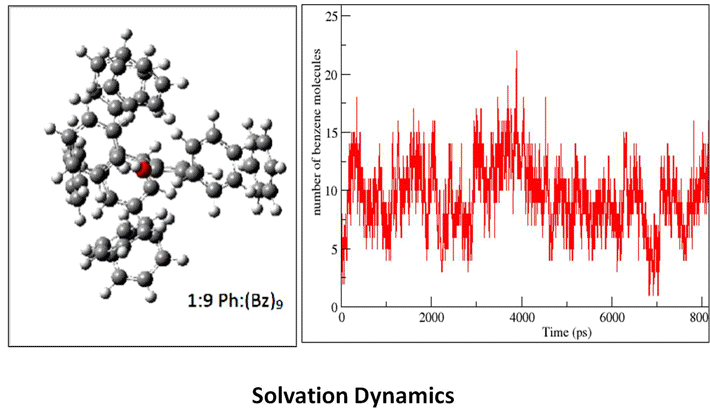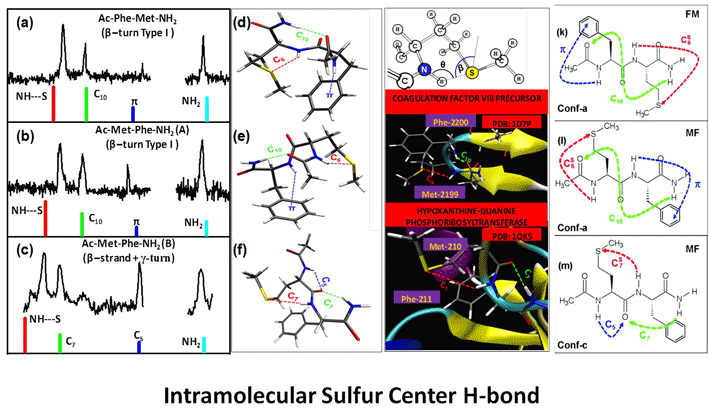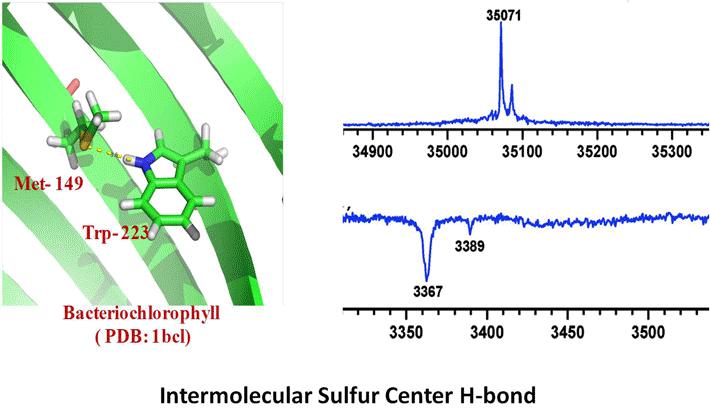
The biological functions of biopolymers such as RNA, DNA, proteins and polysaccharides etc. are mainly regulated by their structures. The inter and intra molecular non-covalent interactions between the monomer units as well as with the solvents controls the conformational landscape of these flexible molecules. X-ray crystallography and NMR are the two widely used experimental techniques to predict their three dimensional structure in the solid and condensed phase, respectively. However, these two methods, especially NMR structure determination heavily depend on the theoretical structure prediction using different force fields. Reliable force fields and good theoretical models are very necessary to corroborate the experimental data. To design a reliable force field, one needs to have molecular level understanding of the non-covalent interactions that govern their structure. This can be achieved by validating the theoretical predictions against the benchmark experimental results. The combination of laser evaporation of biomolecules with sophisticated double-resonance spectroscopic techniques provides many valuable and precise experimental data on isolated biomolecules in general and amino acids and peptides in specific for the benchmarking of theoretical methods. High resolution laser spectroscopy also helps in understanding the conformational preferences of amino acids in small peptides and their role in protein folding. Some of our research interests are briefly outlined. First, we are broadly interested in microsolvation of peptides and bio-molecules in gas phase. A complete understanding of the role played by the environment of a protein on its structure is still far from being achieved. From the theoretical point of view, modeling of a solvated protein chain as simple as the alanine dipeptide analogue (Ac-Ala- NH-Me) is still an open question for molecular dynamics studies [1]. Two dimensional infrared (2D IR) spectroscopy or nuclear magnetic resonance (NMR) of peptides in liquids provide conformational characterization [2,3] as well as few structural parameters that can be used for benchmarking purposes [4]. Alternatively, gas phase spectroscopy experiments provide accurate data but are limited to isolated systems. In order to address solvation issues and still benefit from the accurate measurements of gas phase spectroscopy, our approach consists in studying mixed solvent/solute molecular clusters where the number of solvent molecules in the system is well defined [5, 6]. This page will be updated soon. Please visit our publication page for more information. Selected Publications
>> Next: Non-covalent Interactions
|