Room temperature ionic liquids (ILs), made up of organic
cations and organic or inorganic anions usually possess negligible
vapor pressure and hence have several potential applications as
solvents for synthesis and catalysis besides other industrial
applications. Ionic liquids based on imidazolium cation with long alkyl
chains, form aggregates in aqueous solutions.
Atomistic molecular dynamics (MD) studies have been performed
to study the aggregation in these systems. Starting from uniform
distribution, the aqueous solution of [C10mim][Br] has been found to
spontaneously form the cation aggregates. MD simulations of the
vapor-liquid interface of the above system shows that the surface is
filled with alkyl tails of the cations so as to minimize the
unfavorable interactions between alkyl chains and water. Beyond certain
concentration the cations form aggregates, with polar head groups
(imidazolium ring) at the surface of the aggregates and the alkyl tails
buried deep inside.
The determined aggregate
size agrees with one of the experimentally reported values. Atomistic
simulations of these systems can probe up to several tens of
nanoseconds, which may be a short compared to the timescale of
aggregation process. The simulated system appear to be in a metastable
state with reference to the aggregation numbers. To address this issue
a coarse grained model has been developed for the system.
Coarse
grained MD studies on the aqueous [C10mim][Br] solution at
several concentrations of ionic liquid have provided insights into
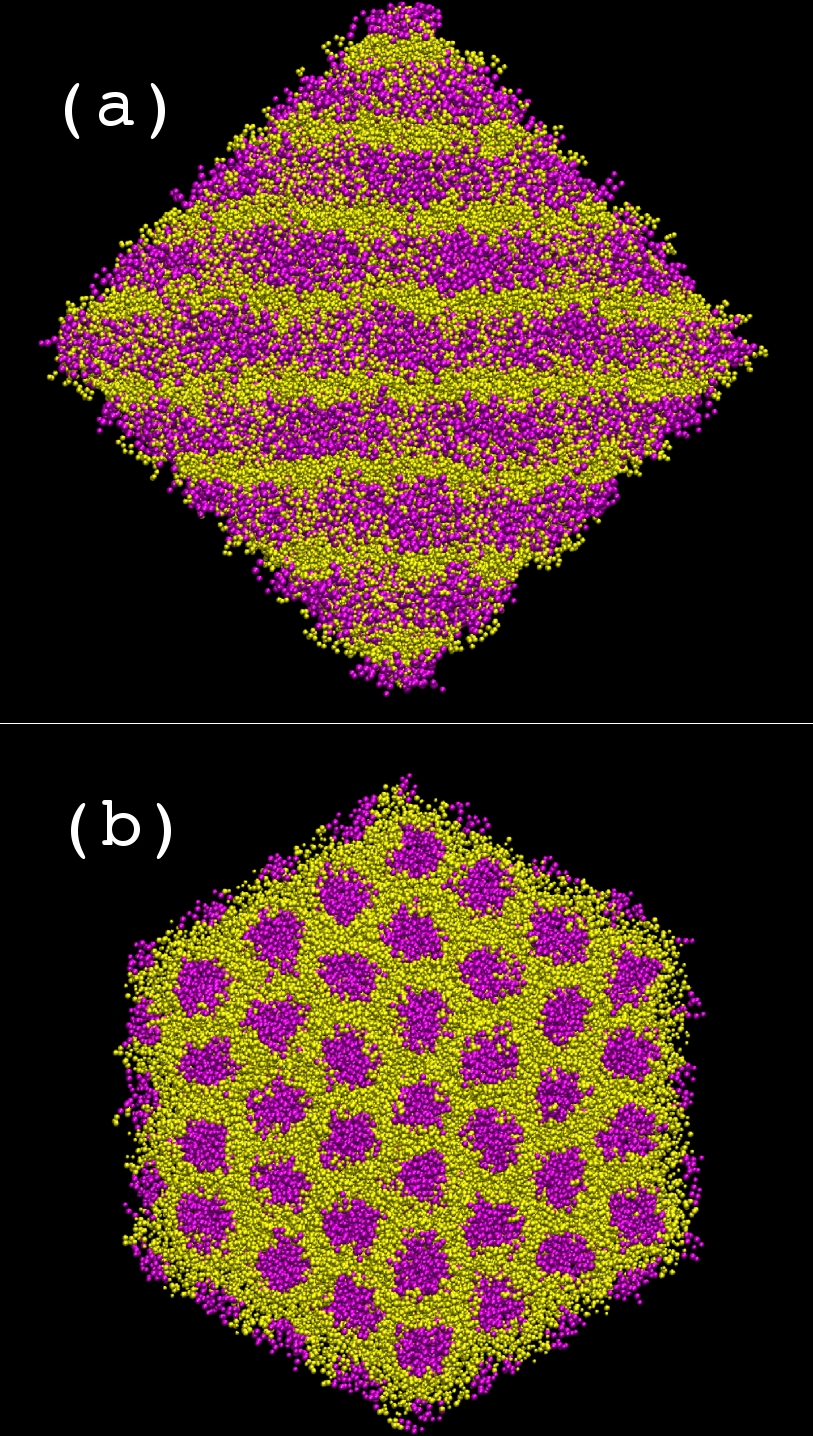
their microscopic structure. The highest concentration studied using
simulations corresponds to 37% (w/w) water. At this concentration there
is a separation of hydrophilic (head groups, anions and water, shown in
yellow) and hydrophobic regions (tail groups, shown in magenta) in such
a way as to form a hexagonal columnar phase (Figure (a) side view of
columns and (b) top view of columns). The spacing between the columns
is 32.1 angstroms in comparison to the experimentally observed value of
33.2 angstroms.
A relatively
dilute aqueous solution at a concentration of 0.2 M of
[C10mim][Br] was chosen to study the micellization process.
Quasi-spherical aggregates with alkyl tails at the core and head groups
at the surface were spontaneously formed in the solution. This
structure allows the head groups to favorably interact with water and
anions while the hydrophobic alkyl tail groups are shielded from the
water. Initially, large number of monomers and few small aggregates
were observed. With the evolution of time, the aggregates grew in size
by adsorbing the monomers.
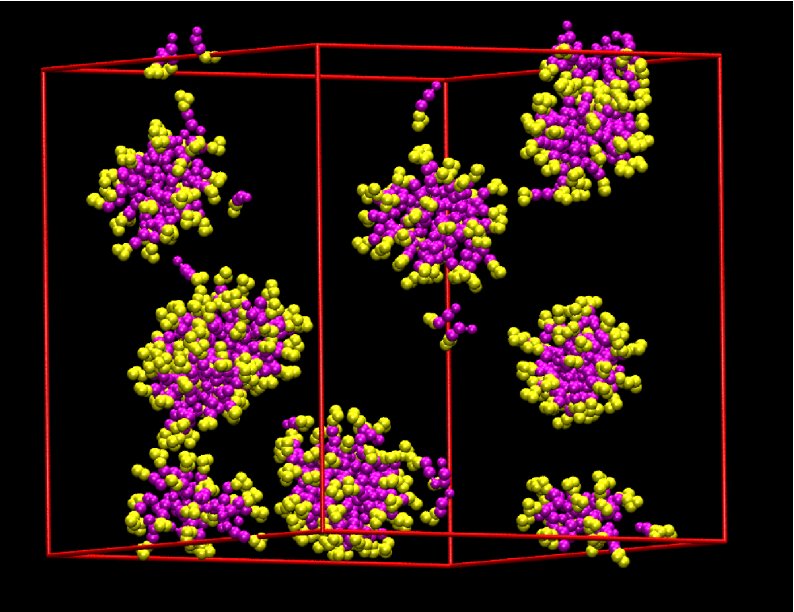
Once
the small aggregates are formed,
it is difficult for these to combine to form larger aggregates due to
the presence of charged surface, which results in double-layer-like
repulsion of the micelles as they approach each other. On rare
occasions, two small micelles approach each other and fuse together to
form large micelles. The observed aggregates were poly-disperse and
most of the cations belonged to the aggregates of size between 40 and
60. The bulk region of the vapor/liquid interface of aqueous ILs shows
similar aggregation behavior. Nevertheless, cations at the vapor/liquid
interface are organized such that the head groups are submerged in the
water and the alkyl tails protruding out along the interface normal.